Acidic Wine & The Table.
What does the term acidic wine mean, and who wants to drink a wine like lemon juice? Here’s a three minute read with the answers.
Most of us enjoy the sensation of freshness that some wines give, whites in particular. This is due to a number of factors, but when we refer to freshness, acidity is the main player. This applies to many other drinks too, and many of Italy’s traditional wines (the ones that have a long history), can often be described as fresh due to their acidic component. This is particularly the case in the Tuscany region whose wines I’ve been brought up on. I guess it’s a matter of habit, but I find it hard to enjoy wines where the acidic component is subdued.
Perceived acidity
Sometimes higher sugar levels can mask the acidity, we all know the difference of a straight lemon juice and one with sugar. The sugar doesn’t alter the acidic concentration, it just makes our taste buds less aware of it. Some wines are purposely made to be sweet, or have a sweet/acid balance. For the most part, these are desert or sipping wines like Vin Santo from Tuscany.
Balanced unbalanced
Apart from those wines which intentionally sweet, a balanced wine is one where acidity, alcohol, residual sugars and tannins interact in perfect way. An unbalanced wine is obviously the opposite. For example too much acidity will result in an excessively sharp wine, while to much sugar will make the wine taste flat like something is missing. Too much tannin will confer a bitter dry sensation. A high alcohol content (which sounds like fun) will overpower all the other flavors. High alcohol levels can also be a trick to make a poor wine seem better.
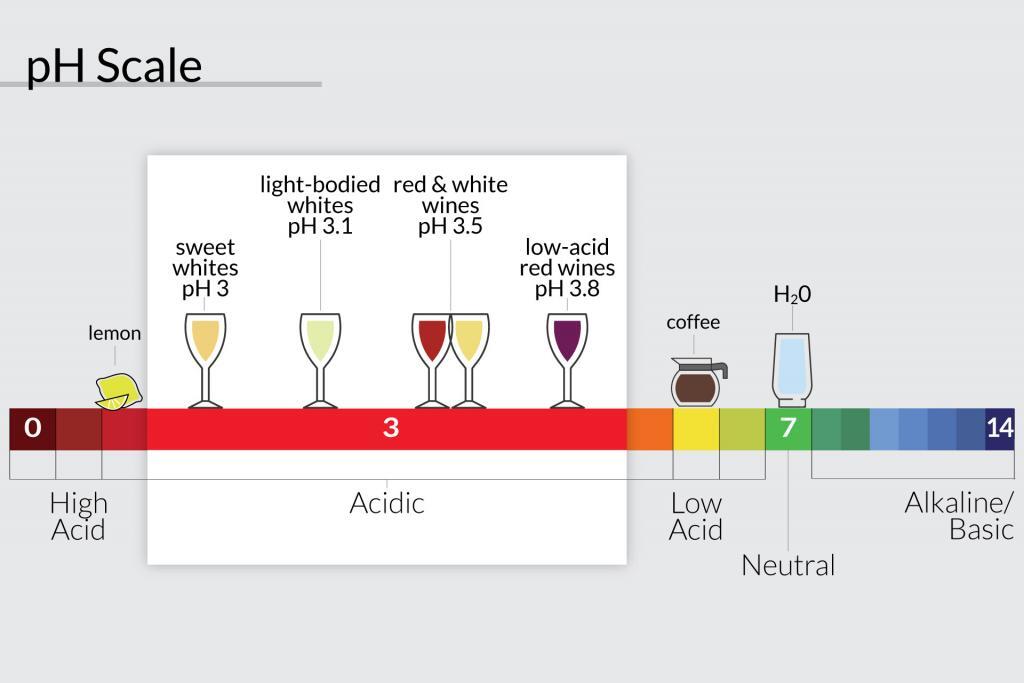
The acidity of Tuscan Wine
My Tuscany wine tours take us the Chianti region and of course to the home of Chianti and Super Tuscan wines. The average pH of these wines is around 3.5 with residual sugar levels of 4 grams per liter. This means you can expect wines with a certain zing and freshness.
So why are Tuscan wines better with food?
Well all wines have a certain amount of acidity. Acidity brings a sensation of freshness, and is a necessary ingredient of the flavor profile. Even desert wines often have a touch of acidity. However the bolder and softer the wine, the lower the initial acidity. The introduction of food and salt on your palate will reduce your perception of acidity even further. So obviously if you don’t have much to start out with, the wine will lose freshness and flavor. When this happens we refer to the wine as being unbalanced.
On the other hand Tuscan wines like Chianti and Brunello will taste fresh and leave your mouth watering. This is because they have a higher acidity to start out with, so there will be more left over after the introduction of food. In other words, the wine retains a better balance.
What is an acid?
I’m not a chemist, so here’s a simple definition that even I can understand. An acid is any substance that in water solution tastes sour.
In more scientific terms an acid is a substance that changes blue litmus paper to red, reacts with some metals to liberate hydrogen, reacts with bases to form salts, and promotes chemical reactions (acid catalysis).
An acid is a chemical substance with pH of less than 7 (see diagram above). The pH is a scale used to specify how acidic or basic a water-based solution is. The scale goes from 0 to 14. Acidic solutions have a lower pH, while basic solutions have a higher pH. At room temperature (25°C or 77°F), pure water is neutral being neither acidic or basic. The pH is 7. Read more on Wikepedia.
The Primary Acids
There are are a quite a number of acids involved in winemaking, and they all have an influence on the final product. Some are positive and some are negative. The primary acids are Tartaric, Malic and Lactic and Acetic. Most of these acids are fixed acids, with the exception of Acetic acid. This is the acid we mostly find in vinegar. Acetic acid is volatile and can contribute to the wine fault known as volatile acidity. Sometimes, additional acids, such as citric, ascorbic, sorbic and sulfurous acids, are used in winemaking.
Tartaric acid
Tartaric acid in wine making is the most important due to the role it plays in maintaining chemical stability and color. It’s also very important for the taste of the finished product. Tartaric acid is a rare in most plants, but the grape vine contains significant amounts. The concentration varies depending on grape variety and the soil content of the vineyard. Sometimes the Tartaric acids can crystallize and may appear like broken glass. They are harmless but winemakers will put the wine through cold stabilization, which causes the Tartrates to precipitate out of the wine.
Malic acid
Malic acid is another organic acid found in wine grapes. It can be found in almost all fruits, and is often associated to green apples. This is also the flavor it tends to give to a wine.
Malic acid in the vine is involved in several processes which are essential for its sustainability. Its concentration varies depending on the grape variety and the time of season. It’s at its peak just before veraison. Then as the season progresses, it’s metabolized with the process of respiration of the vine, and will be very low by harvest time. The respiratory loss of malic acid is more pronounced in warmer climates. When all the Malic acid is used up in the grape, it is considered “over-ripe.”If this happens the winemaker must compensate by adding acid at the winery. This process is known as acidification.
In the case of Tuscan wines such as Chianti and Brunello, the grapes are harvested long before they are over ripe. Therefore the residual Malic acid will be further reduced during through a process known as malolactic fermentation. In this process, bacteria convert the stronger Malic acid into the softer lactic acid. Malolactic fermentation will increase the pH (less acidic), and consequently give a different sensation in the mouth.